Details of the Drug
General Information of Drug (ID: DMUPE07)
| Drug Name |
Nicotinamide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aminicotin; Amixicotyn; Amnicotin; Benicot; Dipegyl; Endobion; Enduramide; Hansamid; Mediatric; Niacevit; Niacinamide; Niacotinamide; Niamide; Nicamina; Nicamindon; Nicasir; Nicobion; Nicofort; Nicogen; Nicomidol; Nicosylamide; Nicota; Nicotamide; Nicotilamide; Nicotililamido; Nicotinamid; Nicotinamida; Nicotinamidum; Nicotinsaeureamid; Nicotinsaureamid; Nicotol; Nicotylamide; Nicotylamidum; Nicovit; Nicovitina; Nicovitol; Nicozymin; Nikasan; Nikazan; Nikotinamid; Nikotinsaeureamid; Niocinamide; Niozymin; Papulex; Pelmin; Pelmine; Savacotyl; Amid kyseliny nikotinove; Amid kyseliny nikotinove [Czech]; Amide PP; Astra Brand of Niacinamide; Austrovit PP; Delonin amide; Factor pp; Inovitan PP; Jenapharm Brand of Niacinamide; Merck Brand of Niacinamide; Niacinamide Astra Brand; Niacinamide Jenapharm Brand; Niacinamide Merck Brand; Niacinamide Pharmagenix Brand; Niacinamide [USAN]; Niavit PP; Nicotine acid amide; Nicotine amide; Nicotinic acid amide; Nicotinic amide; Nicotinsaureamid Jenapharm; Nicotinsaureamid [German]; Nikotinsaeureamid [German]; Pelonin amide; Pharmagenix Brand of Niacinamide; Vitamin B; Vitamin PP; Witamina PP; Nicosan 2; Vitamin H1; B 3, Vitamin; B3, Vitamin; Beta-Pyridinecarboxamide; Jenapharm, Nicotinsaureamid; Nandervit-N; Niacin-Vitamin B3; Niacinamide (USP); Nicotinamida [INN-Spanish]; Nicotinamide (Niacinamide); Nicotinamidum [INN-Latin]; Niko-tamin; PP-Faktor; Vi-Nicotyl; Vitamin B (VAN); M-(Aminocarbonyl)pyridine; Niacinamide, Nicotinic acid amide, Nicotinamide; Nicotinamide (JP15/INN); Nicotinamide, niacin, vitamin B3; Nicotinamide-carbonyl-14C; Pyridine-3-carboxamide; Pyridine-3-carboxylic acid amide; 3 Pyridinecarboxamide; 3-Carbamoylpyridine; 3-Pyridinecarboxamide; 3-Pyridinecarboxylic acid amide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
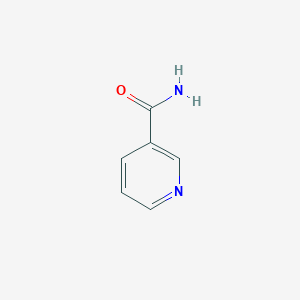 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 122.12 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acne vulgaris | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ED80 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Nicotinamide (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
